Abstract
Introduction
CRISPR/Cas9 system is a highly efficient genomic editing system. However, application of CRISPR/Cas9 system in vivo is somewhat limited for delivery methods and safety concerns. Extracellular vesicles (EVs) are released by exocytosis which results in consistent surface membrane proteins with original cells and capable to deliver several kinds of materials in vivo. However, EVs can be absorbed by other organs or tissues besides tumors, leading to possible off-target effects. Therefore, it is imperative to enhance the targeting property of EVs in vivo. Recently, clinical advances have confirmed that CAR-T cells possess great tropism and multiple choices of target. This may be an effective means to improve EVs' selective accumulation in tumors. In this proof-of-principle study, we exploit the target precision of CAR to establish an EV-derived tropism-delivery platform for CRISPR/Cas9 system as a novel therapeutic strategy for malignant diseases.
Methods
We established CD19-CAR-293T cell line by CAR lentivirus transfection. Raji cells (Burkitt lymphoma) were chosen as target tumor cells for it's CD19 positive. For the isolation of EVs from CD19-CAR-293T and 293T cells, culture medium was harvested, centrifuged and filtered through 0.45μm filters to remove the dead cells and large microvesicles. Purified EVs were examined by (NTA). The plasmids of CRISPR/Cas9 system targeting human cMyc gene were constructed by standard protocols and electroporated into EVs using Gene Pulser Xcell Electroporation System (BioRad). EVs were labelled with lipophilic fluorescent dye for visualization of confocal images. Uptake of EVs in Raji cells was analyzed using confocal microscope. To observe the biodistribution of EVs, Raji-bearing xenograft NOD/SCID mice were generated. When the tumors reached 200mm3, labelled EVs were intracardially injected to mice. Biofluorescence images were obtained using IVIS imaging system.
Results
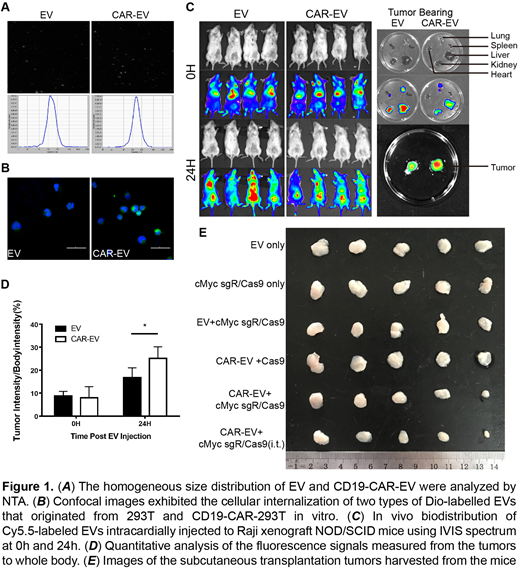
NTA showed that a size distribution of approximately 100nm to 450nm for two types of EVs (Fig. 1A). The modifications didn't affect the physical properties. We used a fixed number ratio of Raji cells to EVs in incubation to compare the uptake efficiency between normal EVs and CD19-CAR-EVs. After one hour of incubation with Raji cells, uptake efficiency of the CD19-CAR-EVs was higher than that of the normal EVs, as indicated by signal intensity in the confocal microscope images (Fig. 1B). To confirm whether CD19-CAR-EVs could target tumors in vivo, we intracardially injected the same number of labelled EVs or CD19-CAR-EVs to Raji xenograft NOD/SCID mice and observed at 0h and 24h post-injection. As results, the CD19-CAR-EVs significantly accumulated in tumor sites compared to liver, kidney and other tissues. In contrast, the normal EVs were distributed throughout the body (Fig. 1C). Ex vivo images exhibited that compared with the EV group, the CD19-CAR-EVs accumulated more in tumors and less in liver, kidney and other tissues (Fig. 1C). The tumor/whole-body fluorescence intensity ratios were calculated in the EV and CD19-CAR-EV groups (Fig. 1D). At 24h post-injected, the ratios in the CD19-CAR-EV group were higher than that in EV group (P<0.05).
Twelve days after the subcutaneous xenograft models were generated, CD19-CAR-EVs loaded with CRISPR/Cas9 system targeting human cMyc were injected intracardially or intratumorally (i.t.) to mice with interval of 4 days for 5 times. Mice injected with EVs only, with plasmid of cMyc sgRNA/Cas9 only, and with Cas9 loaded CD19-CAR-EVs without sgRNA were used as control groups. Tumor sizes were compared among groups at 18 days post the first EVs injection. Tumors from intracardially injected CD19-CAR-EVs+cMyc sgRNA/Cas9 group were smaller than those from EVs+cMyc sgRNA/Cas9 group. And the tumors from intratumoral injection of CD19-CAR-EVs+cMyc sgRNA/Cas9 were the smallest ones among groups (Fig. 1E).
Conclusion
In this study, we take advantages of CAR's affinity to tumor cells and EVs' delivery capacity, and generated CD19-CAR-EVs capable of delivering CRISPR/Cas9 system. This CAR-EV-derived tropism-delivery system can be administered systemically or locally and accumulated selectively in tumor tissues to achieve gene editing in vivo. With evolving repertoires of CARs and multiplex genome engineering abilities of CRISPR/Cas9, this approach is with great potential for biotechnological and therapeutic applications.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal